Introduction
Adequate samples from bone marrow biopsies are required for pathologists to diagnose and monitor hematologic conditions accurately. These biopsies involve the extraction of bone marrow samples in two main forms: an aspirate and a core biopsy. In some cases, additional molecular testing may be conducted for the purposes of diagnosis and classification. The journey of a bone marrow sample from the patient's bedside to the laboratory is a complex process that involves the collaboration of numerous participants and interconnected systems to ensure the integrity and accuracy of the results. At our cancer center, we initiated a quality improvement (QI) project to assess the baseline success rate of bone marrow sample collection, identify areas for improvement in collection, form and implement targeted interventions, and then measure the project's success.
Methods
In this QI study, we utilized an A3 model which included 1) assessing the current state of bone marrow biopsy samples at our institution 2) reviewing the current process of collection 3) analysis to identify the root causes affecting quality 4) Identifying our goal state 5) implementing interventions to improve the quality and 6) follow up to evaluate the effectiveness of our interventions against the stated goal.
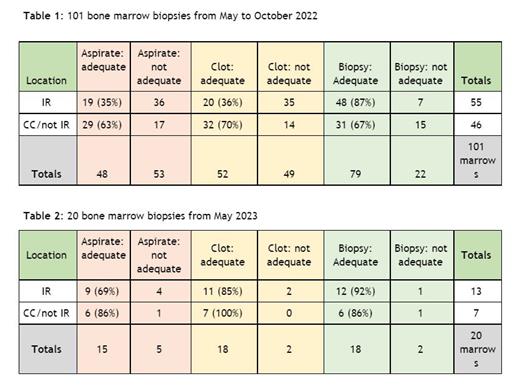
To identify the current state, we assessed the quality of 101 bone marrow biopsy samples collected from May to October 2022, focusing on the aspirate, clot, and biopsy sections. Two pathologists reviewed 20 of these samples at random to re-read them and defined inadequate aspirate and clots as those which were ‘aspicular’ or ‘paucispicular’ and inadequate trephine biopsies as those that were reported as ‘limited’ or ‘inadequate’ for interpretation. This limited analysis was hypothesis generating and we relied on descriptions of aspirate quality and core length in the biopsy reports for the remaining samples. We observed different providers and technicians performing bone marrow biopsies to understand the current process of collection across our institution. Our system analysis identified the following issues: Non-standardized collection technique, communication errors between technicians and providers in terms of core length and aspirate adequacy, and technician proficiency in identifying spicules and core length
To address these issues, we wrote a guideline outlining the recommended standard technique and performed a training for all providers. This guideline included a standard communication guideline between technicians and providers. Technicians underwent additional training in terms of identifying spicules and measuring core length. We set a pre-defined goal of success as 70% adequacy for bone marrow biopsy aspirates and 90% adequacy for core biopsies. Following our interventions, we started collecting post-intervention bone marrow biopsies in May 2023 and plan to evaluate the adequacy of 100 bone marrow samples
Results
Samples were categorized as those performed by interventional radiology (IR) providers and non-IR providers (advanced practice providers, attendings, and fellows).At baseline, the adequacy rates for aspirate, clot, and biopsy sections were 35%, 36%, and 87%, respectively, for IR providers, and 63%, 70%, and 67%, respectively, for non-IR providers. (Table 1)We plan to evaluate 100 samples post-intervention and to date, we have evaluated 20 samples. Among IR cases, adequacy rates for aspirate, clot, and biopsy sections were 69%, 85%, and 92%, respectively. For non-IR cases, adequacy rates were 86%, 100%, and 86%, respectively. (Table 2)
Although additional samples are needed to confirm our results, there is thus far a noticeable improvement in the rate of adequate sample collection for both IR and non-IR providers. While not included in the current analysis, we plan to add patient factors that may affect sample collection such as Body Mass Index or use of sedation in our final analysis to address potential confounders.
Conclusion
Through our QI study, we aimed to enhance the quality of bone marrow biopsy samples at our institution. Preliminary results demonstrate improvements in the rate of adequate sample collection for aspirate and core biopsies following our quality interventions. Future studies will explore the impact of patient and disease factors on sample quality and also assess whether the improved outcomes are sustained at a later point in time with additional follow up.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal